Safety
Vacuum
In the lab course, evacuated glass vessels are used at various points and vacuums are also generated in some cases. Evacuated glass vessels are usually located behind protective screens. Where this is not the case, protective goggles are available at the experimental set-ups. You are urgently requested to wear them.
Electricity
Electricity poses hazards to the equipment used in the lab course (e.g. in the form of destruction by overvoltage, fire, explosion), but also to humans (e.g. in the form of burns, temporary paralysis, ventricular fibrillation). The degree of danger is determined by current intensity, voltage and resistance. Danger to life exists with:
- Direct voltages of over 100 V,
- AC voltage of more than 50 V and 50 Hz.
Safe Handling of Electricity
To minimise your exposure to danger, strictly observe the following safety rules:
- Electrical circuits may only be set up, converted or dismantled when no voltage is applied.
- You may only apply voltage after the supervisor of the experiment has checked the corresponding circuit.
- Switch off all equipment at the end of the experiment.
- In emergencies, switch off the voltage in the relevant practical room immediately. Red emergency switches are located next to each door.
- To protect the equipment, regulate all voltages to zero by hand before switching off the equipment.
Laser
The handling of lasers is associated with unavoidable dangers. These result from the high directionality and intensity of the laser beam, which lead to very high energy surface densities. The hazard potential of a laser can be recognised by its laser class. The lasers in the practical course have the following classifications:
Class 2:
- The laser radiation is in the visible spectral range (400-700 nm).
- The beam power is so low that it is harmless to the eye if the exposure time is short (less than 0.25 seconds), as is the case with the eyelid closure reflex.
-
The maximum value of accessible radiation is < 1 mW.
Class 3R:
- The accessible laser radiation is dangerous for the eye and in special cases also for the skin.
- The beam power can be in the range of 1 to 5 mW.
In the lab course, only He-Ne lasers with emission at a wavelength of 632 nm and a beam power of less than 5 mW are used.
Safe Handling of Lasers
↵
You minimise the hazards of working with lasers if you follow the rules of conduct below:
- Only the students conducting the experiments and the relevant supervisors should be present at the experiments.
- Experiments involving the use of lasers may only be carried out after laser safety instruction has been given.
- Plan the exact set-up of the experiments and determine the steps necessary for adjusting the optical elements before carrying them out.
- It is essential that protective goggles are worn during all manipulations in the area of the beam of a class 3R laser.
- In any case: Never look directly into a laser beam and do not wear jewellery or wristwatches that could reflect laser light uncontrollably when working in the laser area.
Radioactivity
Handling radioactive substances is dangerous due to their ionising effect and toxilogical properties and requires special care. For nuclear physics experiments, the rules of the radiation protection instruction must be followed! Basically, the handling of these substances is regulated by the Radiation Protection Ordinance issued on the basis of the Atomic Energy Act. It prescribes who may handle radioactive substances, when, where, for how long and under what conditions, and aims to prevent unnecessary radiation exposure or contamination of persons, material goods or the environment.
Types of Radiation
Alpha, beta and/or gamma radiation occur during the decay of unstable atomic nuclei. Alpha radiation consists of He nuclei, beta radiation of electrons or positrons and gamma radiation of high-energy electromagnetic radiation. Radioactive decays are stochastic in nature, i.e. they occur randomly. How strongly a radioactive material radiates is characterised by the activity A, i.e. by the number of decays per unit of time. A is measured in units of becquerels [Bq]. One becquerel corresponds to one decay/second. The old unit curie [Ci, 1 Ci = 3.7-1010 Bq] corresponds to the activity of 1 g 226Ra.
Radioactive nuclei occur everywhere in nature. The human body, for example, has a radioactivity of approx. 104 Bq.
Definition of the Dose Measurands
In general, the effect of ionising radiation in matter is described by the quantity dose. A distinction is made between ion dose and absorbed dose. The cause of the radiation effect (e.g. its biological damage) is the deceleration and absorption of primary or secondary charged particles. Gamma radiation in this case generates charged particles through a first interaction.
Ion dose is defined as charge generated per unit mass of biological tissue and is expressed in C/kg. An older unit for this is the X-ray [R, 1 R = 2.58-10-4 C/kg].
The damage caused by radiation in biological tissue is proportional to the energy deposited in the body. The absorbed dose corresponds to the absorbed energy per unit mass assuming a spatially constant energy flux density. It is given in units of Gray (1 Gy = 1 J/kg). An older unit for this is the wheel [rd: 1 rd = 0.01 Gy].
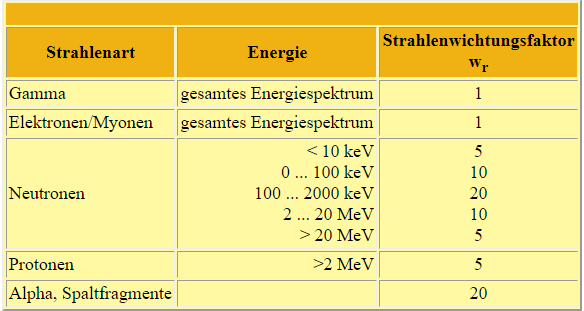
The biological harmfulness of radioactive radiation to biological tissue depends not only on the absorbed dose but also on the type of radiation. This is taken into account by the radiation weighting factor wr, which depends on the ionisation density of the radiation. The product of absorbed dose and wr defines the equivalent dose. This is measured in units of sievert (1 Sv = 1 J/kg). An older unit for this is the rem [rem: 1 rem = 0.01 Sv].
The following table shows the values for wr for different types of radiation, some with different energies:
Shielding From Radioactive Radiation
The highest radiation protection requirement is to keep any radiation exposure as low as possible, even below the legally defined exemption limits. Since the intensity of a source radiating uniformly into space decreases with 1/(distance)2, an effective method of protecting oneself from radiation is to keep one's distance.
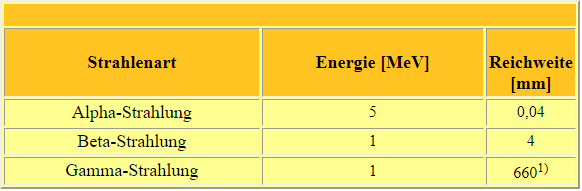
In addition, radiation is weakened or even completely absorbed when it passes through matter. Gamma radiation is absorbed much more strongly by heavy materials (with a high nuclear charge number, such as lead) than by light materials of the same thickness. Beta radiation, on the other hand, is better slowed down by light nuclei, so that bremsstrahlung effects are kept as low as possible. Alpha radiation has very short ranges due to its high ionisation. Even the thickness of a sheet of paper is sufficient to completely decelerate alpha radiation with energies of typical radioactive sources. The following table gives the range of different types of radiation in water (this corresponds roughly to human tissue). In air, the ranges are about 1000 times higher.
Natural and Civilisational Radiation Exposure
The average natural radiation exposure in Germany is about 2.4 mSv per year. It is composed as follows:
- terrestrial radiation: 0.4-8 mSv/year
- cosmic radiation: approx. 0.3 mSv/year
- internal radiation: 1.7 mSv/year
For comparison, we list below some civilisational radiation exposures:
- The equivalent dose during a flight at an altitude of 10 km is about 0.002 mSv/h.
- Medical applications in Germany contribute about 1.5 mSv/year.
- The lethal whole-body dose is approx. 5 Sv. In 50% of cases, death occurs after approx. 1 month after such radiation exposure. By comparison, local radiation exposures of up to 50 Sv can be reached during cancer treatment.
Radiation Exposure Limits
For the protection of the public, areas where radioactive substances are handled must be classified and are subject to a statutory monitoring obligation. This classification is structured as follows:
- Restricted area: Areas in which the local dose rate can be greater than 3 mSv/h.
- Controlled area: Areas in which persons may receive a whole-body dose greater than 1 mSv/year but less than 20 mSv/year. These areas are accessible only to occupationally exposed persons.
- Control area: operational areas where the dose rate limit is guaranteed to be less than 1 mSv/year.
According to §49 StrlSchV, the permissible radiation exposure depends on the individual person:
- Anyone can receive radiation exposure of up to 1 mSv/year in the in-house monitoring area.
- For occupationally exposed persons, this limit is 6 mSv/year for category B and 20 mSv/year for category A (medically monitored).
- Furthermore, the rule applies that half of the annual dose must not be exceeded in 3 consecutive months.
- The accumulated dose in working life shall not exceed 400 mSv.
Dealing With Radioactive Sources
The sources we use in the lab courses are in shielded containers and are usually installed in your apparatus by the supervisor(s). The sources in the lab course are sealed so that there is no risk of contamination or incorporation unless you destroy the housing. Especially the beta sources are very fragile.
If a source housing should be destroyed, the following rule applies:
Do not touch anything and inform the internship supervisor immediately!
Observe the following instructions for handling radioactive sources:
- All nuclear physics experiments may only be carried out after the legally prescribed radiation protection instruction.
- According to §53 StrlSchV, no eating, drinking or smoking is allowed in rooms where radioactive substances are handled.
- Avoid touching the sources with your fingers.
- Always hold the source as far away from the body as possible. However, make sure that you do not irradiate another person.
Mercury
If mercury enters your organism, it is extremely harmful to your health. In the practical course, mercury is mainly used to measure temperatures in thermodynamics experiments. All equipment containing mercury is placed in collecting trays from which it must not be removed. In case of mercury leakage, please inform the organisation of the lab course immediately.
Chemical Solvents
The following summarises general instructions for the safe handling and toxicology of chemicals stored in the chemistry laboratory of the physics practical (room F1-22). A list of the chemicals and corresponding work instructions can be found on site.
The majority of these substances are organic, mostly highly volatile solvents, so that hazards can arise not only from skin contact and ingestion, but also from inhalation of the vapours and through skin respiration.
Harmful Effect
The harmful effects of chemicals on health are mainly due to the fact that they interfere with the chemical reactions of normal life processes. This can lead to damage to cells and organs. When they come into contact with the skin, solvents can dissolve the fatty layer of the skin and thus lead to brittleness of the skin, making it easier for microorganisms and dirt particles to penetrate the body. A number of solvents are absorbed through the skin.
Typical symptoms of acute damage from short-term exposure are
- Drowsiness,
- headache,
- dizziness.
In addition, depending on the substance, organ and metabolic damage may occur. Some solvents are irritating to the skin (reddening and blistering may occur). Vapours sometimes cause irritation of the mucous membranes.
Ingestion of small amounts of solvents over a long period of time can lead to chronic damage. The danger is increased by the fact that ingestion of the substances leads to habituation, which means that damage is often not detected in time.
When handling solvents, it should be noted that their effects on the organism can add up, so that a mixture of different solvent vapours, the individual components of which are still each below the threshold-limit value value in question, can already be dangerous.
Various solvents, especially halogenated substances such as benzene, carbon tetrachloride (carbon tetrachloride), trichloroethylene and trichloromethane (chloroform) have carcinogenic effects. In addition to the physiological properties, the physical properties of many substances must also be taken into account, such as the easy flammability of many solvents (e.g. acetone).
Handling
If possible, you should use organic solvents only with gloves and in a well-ventilated environment. To keep exposure as low as possible, you should observe the following rules:
- If there is a choice between several solvents, use a substance with a high threshold limit value.
- Close solvent bottles immediately after use.
- Use the collection container to discard the leftovers.
- Take care that organic solvents and their vapours do not come into contact with hot objects.
- Do not bring organic solvents into contact with strong oxidising agents (such as alkali chlorates, chlorites or K-permanganate), otherwise there is a risk of explosion.
Halogen-free Solvents
Only halogen-free solvents are used in the lab courses. However, these are flammable and usually ignite easily. Ignition does not necessarily require a flame; it can also be triggered by hot objects whose temperatures are above the ignition temperature of the solvent or by electric flashovers (e.g. in the case of electrostatic charges). Their vapours form explosive mixtures with air, and in some cases a solvent content of 1-2% by volume is sufficient for an explosion. Since the vapours are heavier than air, such concentrations can easily be reached on the floor or in troughs (e.g. in a sink) where they collect. Therefore, you should generally not pour organic solvents down the sink.
Explanation of Terms
We introduce the following terms for clarification:
- Flash point is the lowest temperature at 1013 hPa external pressure at which a liquid forms a vapour-air mixture that can be ignited with a flame.
- The ignition temperature is the lowest temperature of a hot surface at which a vapour-air mixture just ignites.
- The explosion limit is the mixture concentration of vapour and air at which independent combustion can propagate.
- The MAK is the legally permissible maximum workplace concentration of a substance in air.
First Aid
- After inhalation of large quantities: Supply plenty of fresh air and seek medical advice.
- After skin contact: Rinse the affected skin areas thoroughly with water.
- After eye contact: Flush your eyes with an eye wash and consult an ophthalmologist.
- After ingestion: It is best to consult a doctor immediately; it may be necessary to induce vomiting.